
June 2014 – by Khalid Sabihuddin
Clinical research has traditionally been considered to be a branch of medical science focused on better understanding diseases and populations, exploring new potential treatments and better understanding what happens when treatments are made available to a broader population. The classical clinical research paradigm has focused on coming up with a clear and comprehensible research question and medical outcome, using the right study design methods to better help answer the question, run and manage the study, generate outcomes, perform some analysis and then publish the findings. The clear advantages of this model have been well illustrated – a focused approach is taken to a question and a clear response can be produced – the disadvantages, however, are quite numerous. In a surprising, if perhaps expected twist, clinical research as a field has been stuck in a dated paradigm of its own.
Contemporary approaches to clinical research fall short in that there is no requirement to conduct deep and formal reviews of literature. Even more concerning, there is no requirement to truly consider what comes after the completion of research, apart from a publication in a journal. The unfortunate consequence of this approach is that:
– Previous research knowledge and evidence is often ignored or poorly understood;
– Common mistakes and errors are repeated;
– The product of research (new knowledge, findings, etc.) is not necessarily used; and
– Perhaps most importantly, economic and policy needs, which are paramount to the implementation of new evidence, are often considered after the fact or forgotten until it’s far too late.
What do we see as some solutions to these current challenges and shortcomings to the contemporary approach to clinical research?
One solution to the underutilization of previous research knowledge/outcomes is to leverage systematic reviews and network meta-analyses to inform traditional clinical research design. Systematic reviews condense existing bodies of knowledge and provide invaluable insights into lessons learnt from work done previously; network meta-analyses bring the power of combining previously completed studies/trials to permit “…inferences into the comparative effectiveness of interventions that may or may not have been evaluated directly against each other…” (Mills et al, BMJ 2013).
In addition to the informative power of systematic reviews and network meta-analyses, the field of health economics is incredibly powerful in generating information and evidence, using modelling, cost-effectiveness analyses and other methodologies to produce relevant economic information that can be used by policy makers, decision makers, payers, reimbursement specialists and more.
In some ways, the approach that is being advocated is to view research from the lens of product development, with different stages of the product aligning with different aspects of the research continuum (Figure 1).
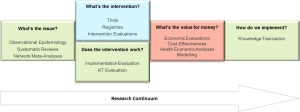
Figure 1: The research continuum from systematic reviews/network meta-analyses, through clinical research, health economics and then ending at knowledge translation
For context – Apple Inc. produces iPhones (the product). Initially, during early generations of the iPhone, Apple would only produce the hardware specifications and the operating system, and would outsource other aspects of manufacturing of the iPhone to third parties. The results of this approach were poor integration, low quality, high cost and poor adoption as few people used the iPhone 1 in a world dominated by BlackBerry. To change this Apple took control of the entire supply chain from design, to manufacturing, through to sales, and either purchased or brought in-house almost all aspects of production.
Much in the same way that Apple vertically integrated their product development, the HUB has successfully brought together expertise in clinical trials, observational epidemiology (including patient registries), health economics, qualitative research, data management, systematic reviews and knowledge translation. This has allowed the HUB to deliver a vertically integrated strategy where knowledge aligns perfectly with the needs of other researchers, payers and policy/decision makers to produce better science and better outcomes at a lower cost.
View Khalid’s profile here or contact us to find out more about the HUB services and expertise.
References:
- Mills, E. J., K. Thorlund, and J. P. A. Ioannidis. “Demystifying Trial Networks and Network Meta-analysis.” BMJ 346.May14 2 (2013): F2914. Web:http://www.bmj.com/content/346/bmj.f2914.
